Introducing onepot
We are building the new infrastructure for molecular creation.
Today we're announcing our automated synthesis lab, and Phil, an AI organic chemist that runs it. onepot's early commercial partners are getting new molecules 6-10x faster, eliminating a critical bottleneck in small molecule discovery.
The problem (almost) nobody talks about
Every drug, every material, every agrochemical starts the same way: someone needs to make a molecule that does not exist. Humanity did that many times, yet, it is still very slow and inefficient.
A typical pharma company waits 6-12 weeks for a handful of molecules. Knowledge does not transfer between projects. Every reaction is essentially starting from scratch, relying on procedures published decades ago in journals behind paywalls with extremely limited reaction scopes reported and without mentioning reactions that did not work.
It is not surprising to see most synthesis projects fail on their first attempt. Quite a few of them will never work. Not because the chemistry is impossible, but because nobody has the data to predict what will actually work. There are 1060 drug-like molecules, but only 122M in PubChem. The largest academic dataset contains only 40k datapoints, the largest industrial one has only 200k.
It is 2025, and chemists are still mixing things by hand, often relying on their intuition and hoping for the best.
We built a different kind of lab
We sat down and looked at the entire compound synthesis cycle to build a different kind of lab.
We use liquid handlers and plate sealers to run reactions in plates. We found that this works for most of the cases, we even have a robot in a glovebox.
But having hardware is not enough, we needed to automate the decision making behind it.
Modelling chemist's intelligence
To automate the decision-making process we did a very unreasonable thing — we gave Phil access to the entire lab. Phil could literally call a tool seal_plate to seal a plate, or write a protocol for liquid handling. He also can easily analyze LC/MS data, look for byproducts, make hypotheses and validate them. We knew that an AI scientist is not a fancy RAG, but a system, capable of executing experiments in the real world and learning from them.
Phil onboarded tens of reactions to our platform by developing protocols and finding optimal sets of conditions. Five of them (reductive amination, Buchwald-Hartwig amination, Suzuki-Miyaura coupling, amide coupling, acylations) are available to our commercial partners, the rest will be available soon. We want the entire community to benefit from Phil's work.
In the past month alone, Phil has run more reactions than a typical graduate student would in their entire PhD. He successfully debugged a number of reactions, for example the following amide coupling experiment:
Phil's analysis of reaction failure
Agent steps
Artifacts
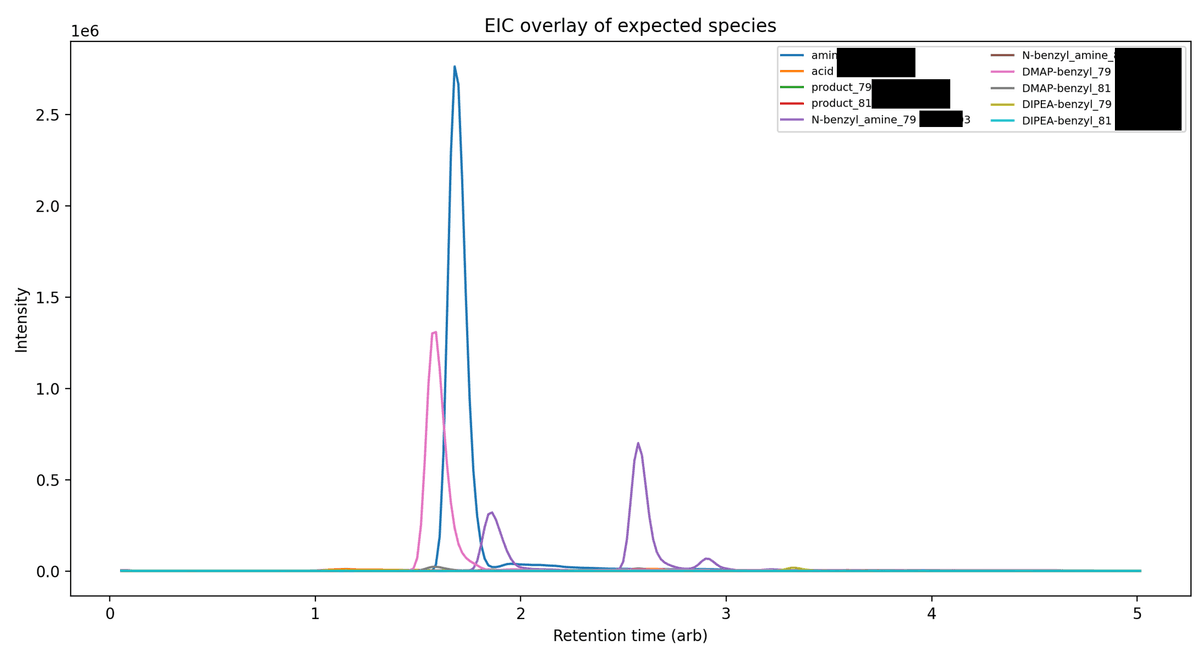
Interpretation: why the coupling failed
The substrate acid contains a benzylic bromide. Benzylic bromides are highly SN2‑reactive and will rapidly alkylate nucleophilic tertiary amines.
Your additives are nucleophilic amines: DMAP (tertiary) and DIPEA (tertiary). The LC/MS clearly shows strong formation of:
- DMAP–benzyl quaternary ammonium salt
- N‑benzylated [...]
- DIPEA–benzyl ammonium
These side reactions sequester DMAP (so it can't catalyze acyl transfer), consume the base, and also convert part of your intended nucleophile (the aniline) into a secondary benzylated amine that no longer couples under these conditions.
Additionally, anilines are relatively poor nucleophiles for amide couplings, and your aniline is [...]deactivated by [...], further slowing the desired coupling. With the key nucleophile/base tied up by alkylation, essentially no productive amide formation occurs. The trace [...] m/z signals suggest only minimal product.
New experiment
When Phil encounters an unexpected byproduct at 2 AM, he does not go home. He designs five new experiments to understand why, runs them in parallel, and incorporates the findings into his next prediction.
Superhuman intuition
Phil can do many more experiments and consider many more ideas, but he has the same limitations as human chemists — often being unable to capture complex structure-activity relationships. To solve that we did three things:
- We collect a lot of data inhouse, including both pretraining-grade (we collected 0 datapoints since you started reading this essay) and higher quality LC/MS traces (just 0 since you started reading).
- Every Phil's experiment is saved to provide an additional source of data.
- We train models on (1) and (2)
So for every Phil's protocol we now know if it is going to be applicable for that particular substrate. This is a hard problem and there is room to grow, but here is a comparison of our model performance against a seasoned organic chemist on a set of hard reactions to evaluate.
We need this intuition not only to know how to synthesize compounds, but also how to purify them. When we rolled out our smart method construction system we saw a huge boost in purity of customer syntheses.
The infinite molecule machine
In five years, waiting weeks for molecule synthesis will seem as archaic as waiting weeks for computational results. Chemistry will become a real-time, iterative science where ideas transform into physical compounds in days, not months.
We are not just automating existing processes. We are creating an entirely new paradigm where:
- Every reaction ever run contributes to predicting the next one
- Synthesis routes are optimized in real-time
- Chemical intuition gets encoded, scaled, and surpassed by machine intelligence
Imagine a world where drug discovery is not bottlenecked by synthesis. Where material scientists can iterate on molecular designs as quickly as software developers push code. Where the 10-year, $2 billion price tag for drug development gets cut in half — through AI-driven chemistry, not luck. This world will be enabled by onepot.
Our supporters
Today, onepot announces $13M in funding from Fifty Years, Khosla Ventures, Speedinvest, Norrsken, and Script Capital, with support from Agata and Wojciech Zaremba (co-founder of OpenAI), Jeff Dean (Chief Scientist at Google), NAVEC, and other visionaries who understand that AI's next frontier is not chatbots — it is atoms.
We are hiring. If you are a chemist tired of running the same reactions your advisor did 20 years ago, a software engineer who thinks molecules are just another data structure, or a robotics expert who dreams of assembling molecules — we want to work with you.
We are also opening our waitlist for forward-thinking organizations ready to compress their molecular discovery timelines by an order of magnitude. Whether you are developing the next blockbuster drug, designing sustainable materials, or pushing the boundaries of chemical space — let's talk.
Visit onepot.ai to explore our platform or join our team.
The future of chemistry is not just about making molecules faster. It is about making molecules that were impossible yesterday, routine tomorrow. Let's build it together.